An Overview of Clinical Trials
Clinical trials are research studies performed in people and test new ways to prevent, detect, diagnose, or treat diseases. They are regulated by agencies, like the Food and Drug Administration (FDA) or European Medicines Agency (EMA) and are the final obstacle in the drug development process.
The FDA defines a drug as any product that is intended for use in diagnostic, cure mitigation, treatment, or prevention of disease; and that is intended to affect the structure or any function of the body. A drug can be a chemical compound, like aspirin, that includes the types of drugs you are most familiar with, or a gene therapy.
The design of a clinical trial, or how the clinical trial is run, often depends on the type of drug being tested and the population that the drug is intended to treat. The most common, or basic, type of clinical trial tests small molecule drugs, i.e., chemical compounds intended to treat a common disease, or medical condition, in a broad section of the population, for example a drug to treat high blood pressure. These clinical trials advance through defined phases of testing with increasing numbers of participants in each phase to demonstrate the drug is safe and effective. This basic clinical trial design is often altered when the intended population is small, such as in a rare disease like STXBP1-RD or when testing gene therapy drugs.
To better understand clinical trials, let’s first look at how classic small molecule clinical trials are designed and run and then compare to clinical trials for drugs like gene therapies or clinical trials run in rare diseases.
Basic clinical trial design
You may already be familiar with, or heard, the terms Phase 1, Phase 2, and Phase 3. Each phase is designed to address specific questions that allow the drug development and approval process to proceed further. These phases are summarized in Figure 1 and expanded on further below.
Figure 1. Phases of a Clinical Trial. Each phase of a clinical trial needs to have sufficient results that a drug is safe and has the potential to benefit patients before continuing to the subsequent phase.
Phase 1
Phase 1 clinical trials are small, meaning that the total number of patients enrolled in the trial is typically between 20 and 80, and can also be randomized placebo-controlled, meaning that the participants are randomized as to whether they will receive the drug or a placebo (a non-active substance, like a sugar pill). These trials usually last weeks to months and because the number of participants is low, these trials are often performed at one or few clinical sites. The goal of a Phase 1 clinical trial is not to see if the drug works but rather if it is safe. Phase 1 clinical trials are often dose-escalation trials, meaning that different groups of participants receive different doses of the drug be tested; usually 3 doses, a low, middle, and high dose. These doses are determined based on data obtained in preclinical studies.
Phase 1 trials help determine what a drug’s most frequent side effects are and the doses at which these side effects appear. Phase 1 trials usually progress in a stepwise manner. An initial group of subjects receive the lowest dose and are observed for any adverse reactions to the drug at that dose. If adverse reactions are absent or minor, then another group of subjects is given the next highest dose and observed (thus the dose escalates). This continues until all doses are tested or until the adverse effects become too great to increase to the next higher dose. Often the lowest doses tested are ‘subclinical’ doses, meaning that they are doses that would not be expected to have any effect on the participant whereas the higher doses are clinical doses, which would be expected to have an effect.
Because the goal of a Phase 1 study is safety, it does not necessarily need to involve people who suffer from the disease the drug is meant to treat. Phase 1 trials often use healthy volunteers because a drug’s side effects can still be observed, but these side effects are expected to have less impact in a healthy person compared to people suffering from a disease.
Phase 2
Phase 2 clinical trials are larger, generally including tens to hundreds of participants (depending on the size of the targeted population) and are usually randomized placebo-controlled. Phase 2 trials can last months to a couple years and are usually performed at a few clinical sites. The goal of a Phase 2 trial is to obtain preliminary evidence if the drug works is effective in people who have the disease the drug is meant to treat. The effectiveness of the drug is generally determined by comparing clinically-measured disease outcomes of people who received the drug to those people who received the placebo. Additionally, safety data, including any adverse effects of the drug continue to be collected.
Phase 3
Phase 3 clinical trials are even larger than Phase 2 trials and can include thousands of patients. Phase 3 trials usually last years and are conducted at multiple sites across a country or in multiple countries. The goal of a Phase 3 trial is to gather more information about drug safety and efficacy in a broad selection of the population.
Phase 3 trials are designed to be large enough to provide regulatory agencies, such as the FDA, with enough data on drug safety and efficacy to allow them to approve the drug for sale and widespread use, this called ‘market authorization’. As data is collected during these trials, changes may be made to the drug, or different subgroups of patients may be tested. It is not unusual for a drug to go through several Phase 1, Phase 2, or Phase 3 clinical trials before it obtains market authorization.
Phase 4
After a drug is approved, safety and efficacy data for that drug are still collected by regulatory agencies. This is sometimes referred to as Phase 4. This data is collected not only from patients who may have been enrolled in the previous Phase 1 through 3 trial but new patients as well. The goal is to obtain long-term safety and efficacy data. The FDA can withdraw a drug’s market authorization if long-term observations uncover safety concerns.
Clinical trial differences in rare diseases
Clinical trials involving rare diseases like STXBP1-RD generally follow the same design principles as described above but allowances are made due to the smaller population size and the appreciation that most rare diseases involve a greater disease burden on the affected population. While some rare disease clincal trials will employ the same randomized placebo-controlled design as used for clinical trials in large populations, many rare disease clinical trials will not as there are insufficient number of patients to assign to a separate control group, and, in the case of life-threatening rare diseases, treating participants with a placebo could be considered unethical. Instead, single-arm clinical trials, in which every participant is given the drug, are often used for rare disease clinical trials. To determine if the drug is effective, the participant population can be compared to historic control data, as obtained in a natual history study. Sometimes participants are followed for a period of time before they receive the drug, this is called a lead-in, and allows the researchers to evaluate the severity of the disease in a given patient before the patient is given the drug. The severity of the disease can then be compared in the patient before and after drug administration to determine if the drug is effective. Single-arm trials still allow for a dose-escalation Phase 1 trial; however, usually the lowest dose tested is a dose that is still expected to have a beneficial effect on the patient. Phase 1 trials in rare diseases usually involve 5-20 participants, depending on the total rare disease population and the type of drug being tested.
Often, for rare diseases, Phase 1 and Phase 2 trials are combined into a single Phase1/2 Clinical trial. In this instance the trial begins with a dose escalation approach, like a Phase 1 trial, and then when the appropriate dose is chosen, additional participants are recruited to expand that particular group and drug efficacy data is collected. In this type of study, the total number of participants will usually be in the tens, usually less than 50. For rare diseases that have obtained orphan drug status it is possible that a drug can be approved by a regulatory authority without having perform an additional Phase 3 trial. This depends on the severity, or life-threatening, nature of the rare disease and the effectiveness and safety that the drug demonstrated during the initial phases of testing. Regardless, if a Phase 3 trial is performed, these trials are still generally small.
Clinical trial differences for gene therapy products
Clinical trials involving gene therapy products can be conducted in either large or small populations, though the majority of gene therapy products are developed for rare disease populations, thus the same restrictions that apply to rare disease clinical trials generally apply to gene therapy clinical trials. The major trial differences associated with gene therapies are that these trials are focused on safety. This is because gene therapies are designed to be permanent treatments, that is, they either introduce a gene into a patient’s cells or modify the patient’s own genes. Unlike small molecule drugs, this type of gene therapy cannot be removed if the patient has an adverse response. Because of this, Phase 1 gene therapy trials usually start with one patient, who is treated with a low dose of the gene therapy and that patient in followed for a one to three months to see if they have any adverse reaction before a second patient is treated with a low dose; a third patient may be treated with a low dose 3 months after the second if no adverse reactions have been observed in either the first or second patient. Regulatory agencies, like the FDA, will usually examine the safety data from the low-dose patient group before allowing a higher dose of the gene therapy product to be tested. Major safety issues that are of concern are adverse immune reactions to the gene therapy viral vector and vector-related liver toxicity. Important differences in Phase 1 gene therapy trials and traditional small molecule trials are that gene therapy Phase 1 trials cannot be conducted in healthy volunteers and the lowest dose tested has to be a dose that is still expected to have a beneficial effect on the patient. Additionally, many gene therapy trials do not include a true placebo control group, for a variety of reasons.
Once the short-term safety of a gene therapy product is confirmed, Phase 2, and if needed, Phase 3 trials can proceed more quickly without long wait times between patients. Regardless, safety is still a primary concern as all patients are subject to long-term follow-up that can range from 5-15 years.
Gene therapy trials that involve the use of antisense oligonucleotides (ASOs) or RNA therapies may not need to meet as high a safety threshold as gene therapy trials involving gene addition or manipulation. This is because ASO and RNA therapies are not permanent and adverse reactions to these types of therapies can be mitigated by lowering the dose or stopping the drug. Still, as these types of gene therapies are new and novel, regulatory agencies remain cautious and Phase 1 trials tend to involve fewer patients and take more time than traditional small molecule trials.
Table 1 below highlights the differences between clinical trials for traditional small molecule drugs for a large population, rare diseases, and gene therapies
Clinical trial participant eligibility
There is one more matter I want to discuss concerning clinical trials, and that concerns eligibility of clinical trial participants. The purpose of clinical trials is to demonstrate that a drug is safe and effective in a particular population at a particular point in time of their disease process. To obtain the cleanest and clearest data, it is important to decrease “noise” or variability in the collected data as much as possible.
To do this, clinical trials employ inclusion and exclusion criteria when choosing participants. Every clinical trial has a set of criteria that participants must meet in order to be enrolled in the study and a set of criteria that will exclude someone from participating in the study. These criteria usually fall within certain categories such as demographics, lifestyle, gender, symptoms, co-morbidities, or other interventions.
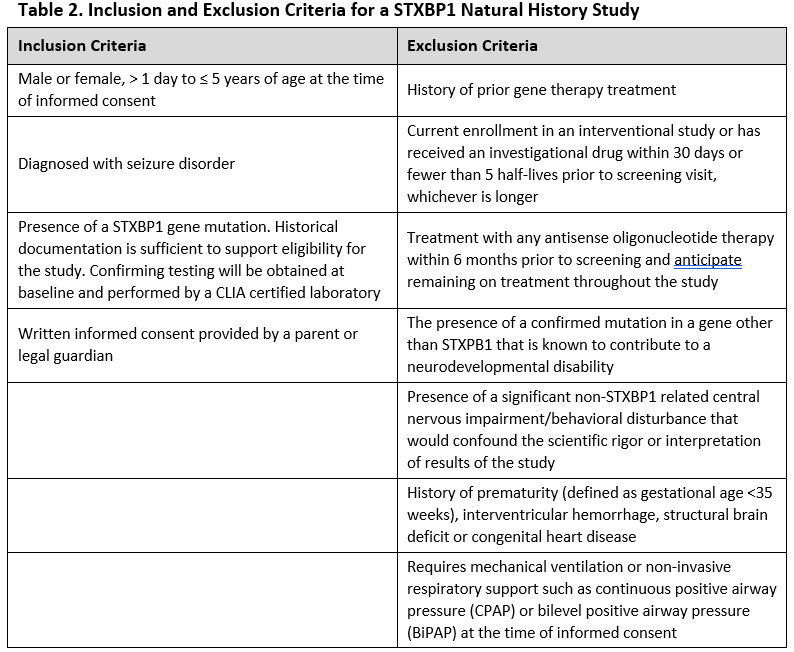
For example, for STXBP1, a clinical trial may restrict the participants to a particular age range where it is felt the drug would have its greatest effect. To a particular area of the country, to make sure the participants can be seen in the clinic running the study. They may require participants be diagnosed with particular symptoms, like seizures. A clinical study may specifically exclude STXBP1 patients who have mutations in genes other than STXBP1 or patients who have other medical conditions such as heart disease. Table 2 lists the inclusion and exclusion criteria for a STXBP1 natural history trial; note that this study is not a clinical drug trial, but similar criteria might be expected for a drug trial.
It is important to understand that if a patient is excluded from a clinical trial, it does not mean that they will be excluded from any eventual treatment with that drug, should it be approved. Additional trials are often performed to expand the inclusion criteria to open up the drug to more subsets of patients. Additionally, if a drug is given market authorization, it could still be used by patients who did not necessarily fit the inclusion criteria of the clinical trials.
Clinical studies are complex, highly regulated endeavors, which I’ve only been able to scratch the surface of in this overview. Still, I hope you’ve gained a better understanding their important in drug development and how. Choosing to take part in a clinical trial is a very important decision, but one I hope you will consider in the future.