STXBP1 Fast Forward - 3 Year Plan
2023 – 2025
The role of STXBP1 in disease was only discovered in 2008, and the STXBP1 Foundation established in 2017. Despite the newness and rarity of our disorder, research is progressing quickly and we are optimistic that viable therapeutics are possible because of these four important learnings:
The root cause is known - changes in the STXBP1 gene cause STXBP1 disorder
We have a strong understanding of STXBP1’s biological function in the brain
We have a much better understanding of the clinical picture of STXBP1 disorders now than we did even 3 years ago.
Restoring levels of the wildtype STXBP1 protein reverses some disease-relevant symptoms in mice
Our Fast Forward Strategic Plan has been published!
Read STXBP1: fast-forward to a brighter future - a patient organization perspective in the journal Therapeutic Advances in Rare Disease.
In 2019 at the foundation’s first scientific meeting, the team mapped out four roadmap priorities to advance STXBP1 research. Following this initial roadmap, and with the amazing work of our research and family community, we have made tremendous progress.
In the three years from 2020 - 2022, we developed a disease concept model, multiple natural history studies, launched an early phase clinical trial for 4-phenylbutyrate, and at the end of 2022, more than 9 potential therapies were in development for STXBP1 disorders.
The therapy pipeline for STXBP1 disorders continues to expand. In Q4 2024 — halfway through our STXBP1 Fast Forward 3 year plan — there are more than 16 potential therapies in development. Two of these therapies have been awarded orphan designation or orphan drug designation. This great progress highlights that we are on track with STXBP1 Fast Forward.
STXBP1 Fast Forward
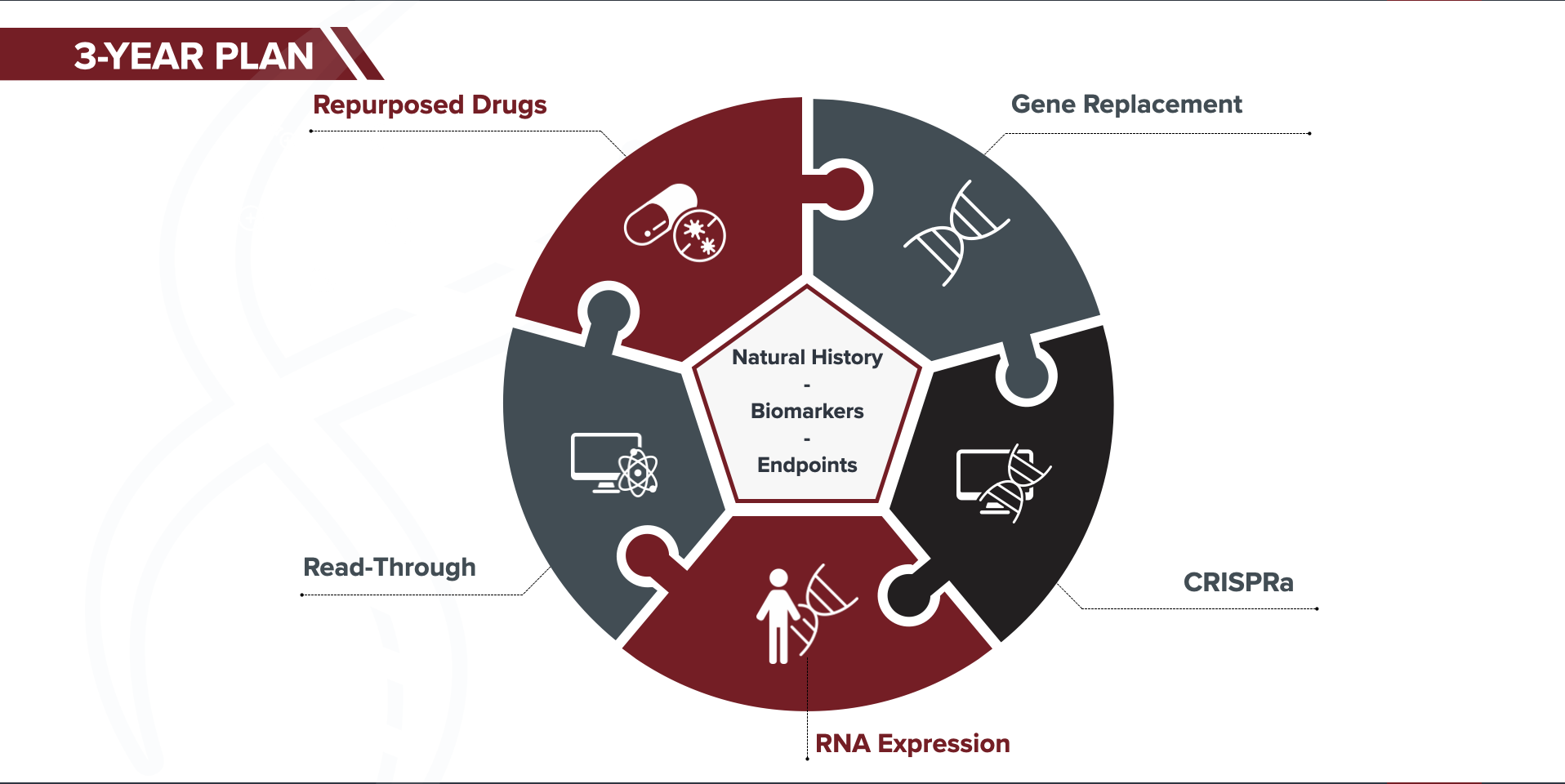
We developed the STXBP1 Fast Forward three-year plan based on the above key learnings, and the current state of the therapy pipeline for STXBP1. This plan builds on the foundation’s key role as a connector and driver within the STXBP1 community. Moreover, we aim to establish and facilitate open sharing of data, reagents and models to accelerate research within the community and to the benefit of our patients.
-
We are aggressively pursuing five therapeutic strategies for STXBP1. Pursuing multiple strategies at the same time gives us more “shots on goal” for effective therapies, and reduces risk.
-
With multiple therapies potentially entering clinical trials in the next couple years, it is imperative that we maximize the likelihood that clinical trials are successful – and that we see improvements in the symptoms that are important to our patients and families. We are leading and orchestrating a number of clinical trial readiness projects:
endpoint and biomarker development
engaging with the FDA to educate them on STXBP1 and what is important to patients.
-
We need to build awareness and interest from biotech and pharmaceutical companies, as they will lead work to commercialize a cure for STXBP1. Through our research and our clinical trial readiness initiatives, we are making STXBP1 an attractive condition for companies to invest in and build therapies for.
-
We are focused on enabling the precision therapies of tomorrow, but we also realize it’s critical to support our patients and families now. We are broadening access to clinical expertise for STXBP1, both through clinical centers for STXBP1 and by developing a standard of care for STXBP1 patients.
Now Is The Time For The STXBP1 Community To Activate
If there are already some pharmaceutical companies developing drugs for STXBP1, do we need to do anything else? The answer is YES!
Our community knows STXBP1 better than anyone else. We need to inform clinical trials with what matters to patients, and drive the development of endpoints that matter
Any single therapy can fail in development or in clinical trials. We must encourage and support as many therapy programs as possible to maximize the likelihood of developing effective therapies and to minimize the downside of any individual therapy failing
A single therapy can be deprioritized by a biopharma based on internal prioritization or market conditions. We need to continue to highlight that STXBP1 is an attractive target, strengthen and build industry relationships, demonstrate our community is activated, and truly understand the size of our patient population
Potential therapies can be initially developed in academia, but they then need an industry partner for later development and/or clinical trials. Our efforts to understand STXBP1 and develop clinical trial readiness de-risks this transition and makes STXBP1 attractive to industry