Chemical Chaperone Therapies for STXBP1
In 2010, Dr. Hirotomo Saitsu linked STXPB1 gene mutations to early infantile epileptic encephalopathies. Dr. Jacqueline Burre and her lab at Weill Cornell further study the mechanisms by which abnormalities in our nervous system, such as mutations to the STXBP1 gene (also called Munc18-1) can trigger neurological disorders.
In 2018, Burre’s lab team published a paper titled “Mechanism-based rescue of Munc18-1 dysfunction in varied encephalopathies by chemical chaperones”, which identified potential “chemical chaperone” therapies for STXBP1. This blog post by Kerry Gao at Weill Cornell Medicine explains this paper.
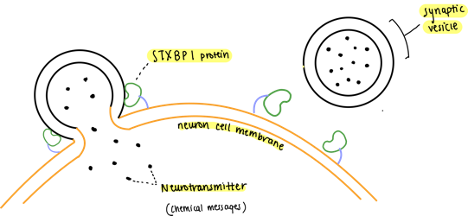
STXBP1 protein plays a key role in our nervous system. STXBP1 proteins assist synaptic vesicles in releasing neurotransmitters to their correct location, i.e STXBP1 proteins aid neurons in our body’s communication by helping relay signals throughout our nervous system.
Analogy: Mailman delivering mail. The mailbag would be the synaptic vesicle that holds all the mail or messages (neurotransmitters). Each post office is a different neuron that must receive the mail. Once the mailman reaches the post office, he must open his mail bag to deliver the mail using his hands (STXBP1 protein).
With mutated STXBP1 protein, there is severely reduced neurotransmitter release resulting in severe neurological impairments such as epilepsies and movement disorders.
For years, haploinsufficiency was thought to be the main disease mechanism of mutated STXBP1.
Through their work in the laboratory, Dr. Burre and her team discovered that mutated STXBP1 was further associated with the following:
1. Destabilization and clumping of the mutated STXBP1 protein.
2. Mutated STXBP1 protein interferes with the remaining normal STXBP1.
As described in the Guiberson et al. 2018 paper, members of the Burre lab found that chemical chaperones could fix these issues. Chemical chaperones are molecules that can improve stability and organization of proteins found in our body.
Analogy: Origami. Proteins in our body must fold into the correct shape to be functional. There are some proteins that need help folding into the correct ‘origami’ structure. Special proteins called molecular/chemical chaperones assist the folding process.
The Burre lab focused on testing chaperones known to stabilize protein structures and/or prevent protein clumping. Laboratory experiments found the following promising chaperone candidates:
4-phenlybutyrate
Sorbitol
Trehalose
These chemical chaperones returned functional STXBP1 protein back to normal levels, reduced seizures, and returned motor function to their live models.
Chaperone therapy is an incredibly promising new therapy, but there remains a further need for research. For the chemical chaperones identified by Jacqueline Burre’s lab, 4-phenylbutyrate was selected to test in a clinical trial at Weill Cornell. 4-phenylbutyrate is already an FDA approved drug for a different condition, urea cycle disorders, and it can reach the brain.
Learn More
Read about the ongoing clinical trial using glycerol phenylbutyrate (ravicti) as chaperone therapy for STXBP1 and SLC6A1: https://clinicaltrials.gov/ct2/show/NCT04937062.
Please visit this link for current ongoing STXBP1 research: https://www.stxbp1disorders.org/current-research.