An Introduction to Gene Therapy
The term ‘gene therapy’ is a catch-all phrase for various types of therapeutic approaches that use or interact with genetic material to treat and/or prevent disease.
However, before I discuss what these various types of therapy are, let's have a little refresher on what I mean by ‘genetic material’.
Genetic material generally refers to DNA and RNA. Recall that genes are made up of DNA and they function as blueprints for making proteins. Each person typically gets two copies of each gene from their parents. RNA helps convert the blueprint instructions contained in the DNA into a format that a cell can use to make the protein. Proteins are what makes our cells and body function. Sometimes a gene is not constructed correctly and may contain a change in the DNA, this is called a mutation and the change can alter the way a protein is made and functions.
Types of Gene Therapy Approaches
Gene therapy has been around for over four decades and during that time there have been a multitude of gene therapy approaches developed and tested, far more than could adequately be covered in this blog. However, the majority of gene therapies approaches can be categorized into four broad categories,: 1) gene replacement/addition; 2) gene editing; 3) cell therapy; and 4) RNA therapy.
Gene Replacement/Addition
Probably the earliest therapeutic approach to be termed ‘gene therapy’ is gene transfer or gene addition (Figure 1). This means putting a replacement gene into a cell to take over the function of a broken gene that is causing a disease or putting a gene that expresses a helpful protein into a cell to fight a disease.
Figure 1. Gene Replacement/Addition. A functional gene is added to a cell, often using virus-based vector delivery, in order to take over the function of a mutant gene or to express a protein that might be beneficial to the cell. In this type of gene therapy the added gene usually remains separate from the patient’s DNA. All figures adapted from: Prondzynski et al, Pflugers Archiv - European Journal of Physiology, 471:807.
For example, STXBP1 disorder is caused by a mutation in one of the STXBP1 genes. This mutant STXBP1 gene cannot make functional Stxbp1 protein, therefore the cell only has about 50% the normal amount, a situation called ‘haploinsufficiency’. One possible therapy is to add an additional normal copy of the STXBP1 gene into cells. This normal copy would make additional Stxbp1 protein, thus compensating for the reduced amount of protein caused by the mutant gene. In this example, it is important to understand that the mutant STXBP1 gene is still present, but its job is being taken over by the new normal copy of the gene put into the cell.
Alternatively, this approach can be used to introduce a helpful gene into a cell to protect it from being harmed due to a toxin or disease process. For example, people who receive some types of chemotherapy to treat cancer develop a condition known as sensory neuropathy, where the feet and hands either become numb or have a constant feeling of ‘pins and needles’. This is caused by the toxic nature of the chemotherapy compound. By using gene addition, a protective protein called a neurotrophic factor can be introduced to neurons, which make them resistant to the toxic effects of the chemotherapy.
Gene Editing
Another approach or type of gene therapy is known as ‘gene editing’. This is a newer approach compared to gene transfer or replacement. The goal of gene editing is to treat a disease by changing the existing DNA in a cell.
Gene editing works in 4 different ways: 1) gene editing can fix a mutation in a gene so it functions properly; 2) gene editing can turn on a gene, or make a gene ‘work harder’, that is, make more protein, to help fight a disease, this is called ‘over-expression’; 3) gene editing can turn off a gene that is not functioning properly, this is called gene silencing; and 4) gene editing can remove a piece of DNA that is either impairing normal gene function or is itself causing a disease.
For example, in STXBP1 gene editing could be used to fix a patient’s specific mutation, thus restoring the function of their previous mutant gene (see Figure 2). It could be used to make the other normal STXBP1 gene generate more normal Stxbp1 protein. Or, if a mutation in the STXBP1 gene resulted in a mutant protein that wasn’t just non-functional but was actually toxic to the cell, you could use gene editing to either cut out and remove the mutant gene or stop the mutant gene from making the toxic protein.
Figure 2. Gene Editing Approach for Repairing a DNA Mutation. Gene editing is a gene therapy approach that interacts directly with the DNA present in the cell. One type of gene editing using a set of molecular tools known as CRISPR-Cas to repair DNA mutations, thus allowing a previously mutant gene to now make a normal functional protein.
Unlike gene transfer, which is accomplished by adding new genetic material, i.e. a replacement gene, gene editing is accomplished by adding what are called ‘molecular tools’ to a cell. A discussion of the types of molecular tools and how they work is beyond the scope of this talk, but one molecular tool you might have heard of is called ‘CRISPR or CRISPR-Cas’. So, if you hear someone talk about CRISPR, they are usually talking about gene editing.
Cell Therapy
A third type of gene therapy is what is known as cell therapy. In this type of treatment, a patient’s cells are removed from their body and a new gene is put into the cells, or a faulty gene is corrected, and then the cells are put back into the person (Figure 3). This type of therapy is generally performed on blood cells and is mainly used to treat cancers or disorders of the immune system and thus not a good candidate to treat STXBP1 disorder.
Figure 3. Cell Therapy. Cell therapy is commonly performed by removing a patient’s cells, altering them by adding a therapeutic gene, and then transplanting them back into the patient. This type of therapy usually requires that the therapeutic gene be integrated, i.e. spliced, into the DNA of the patient’s cells.
RNA Therapy
A fourth type of gene therapy approach, and one which is currently enjoying a lot of attention from scientists and physicians, is RNA therapy. RNA therapy refers to the treatment or prevention of diseases by focusing on RNA instead of DNA. If you’ve had a Covid vaccine, chances are you’ve had RNA therapy as the leading Covid vaccines are made from RNA.
There are many types of RNA therapy, again, too many for the scope of this talk, but in regard to STXBP1 disorder, we are generally concerned with what are known as antisense oligonucleotides, or ASOs.
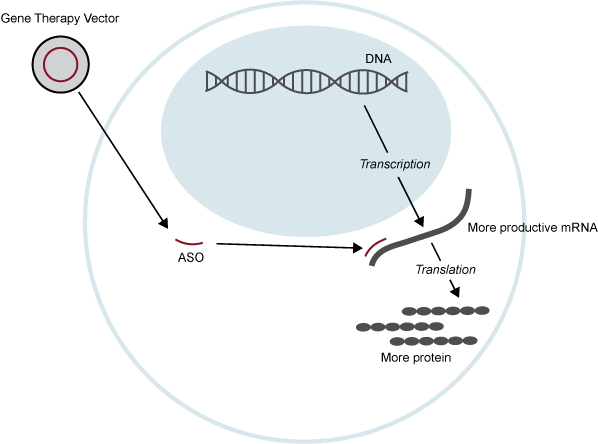
ASOs are small, synthetic pieces of RNA designed to interact with cellular RNA. This interaction can have many consequences, including, ‘silencing’ an RNA so it cannot be used to make a protein, changing an RNA that doesn’t make a functional protein into one that does, or by allowing an RNA to make more protein than it normally would (see Figure 4). This last approach, in particular, could be effective in treating STXBP1 disorder.
Figure 4. RNA Therapy. RNA Therapy is a gene therapy approach that either utilizes RNA as the therapeutic product or interacts with RNA in some way to induce a therapeutic effect. In this illustration, an antisense oligonucleotide (ASO) is delivered into a cell. The ASO ‘attaches’ to a specific RNA and allows that RNA to be used to make more protein than it normally would.
Gene Therapy Vectors
You may have noticed the number of times I mentioned putting something into a cell but so far I haven’t said how that’s done. Genetic material, like replacement genes, and molecular tools, like CRISPR, can be considered ‘gene therapy packages’ and they need a way to be delivered to the specific tissues or cells that you want to treat.
For some gene therapy packages, like ASOs, you could directly inject them into the proper tissues you want to treat; however, for most instances this isn’t possible. In this case you need to use some sort of vehicle that can deliver the package to the proper location. This is performed by what are known as vectors. You can think of a vector as a mail truck that delivers the gene therapy package to where you want it to go.
The most commonly used vectors are based on viruses. Viruses have evolved very efficient ways of finding their way into cells, which make them excellent vectors to deliver a gene therapy package. To use a virus, most of the virus’ genes are removed, so the virus cannot cause a disease itself, and the gene therapy package is inserted into it. The viral vector, containing the gene therapy package, can then be injected into a person.
The most commonly used virus for making vectors is adeno associated virus, or AAV, as it is capable of delivering a gene therapy package to many different types of cells and can be manipulated to deliver a gene therapy package to specific types of cells, such as neurons for treating STXBP1.
Gene Therapy Challenges
Now any discussion of gene therapy wouldn’t be complete if I didn’t also mention some of the problems and challenges.
The most significant issue associated with gene therapy drugs, which have been used in people, involves liver toxicity. One very important job our liver does is that it filters our blood and removes toxins, like alchohol, and foreign substances, like drugs. Those gene therapy drugs that are injected into the blood end up going through the liver and can overstimulate it as they are foreign substances. In rare instances this has caused liver damage in some individuals and in the worst cases a couple of deaths. This problem is being addressed in a couple of different ways. First, the liver is monitored closely in people who receive gene therapy drugs and steroid drugs are given if it looks like the liver is starting to become damaged; this almost always reverses any damage. Second, there is a lot of research currently being conducted on designing gene therapy vectors that can ‘hide’ from the liver. It is very likely that this liver toxicity will be much less of a concern for the next generation of gene therapy drugs.
Gene therapy drugs, which you can think of as the gene therapy package and the vector used to deliver it, are very complex making them difficult and expensive to make. In addition, the cost to get a gene therapy drug approved by the FDA is about 5 times more than for standard types of drugs; one estimate has put this cost at $5 billion. These issues make gene therapy drugs very expensive. Zolgensma, a gene therapy drug for a disorder known as spinal muscular atrophy costs $2.1 million for a one-time treatment. This is obviously much more than the average person could possibly pay. As more and more gene therapy drugs become approved it will be challenging to find ways to pay for these treatments.
Another problem associated with virus-based vectors in particular is that they can promote an immune response in people. This can limit the number of times a person could be treated with a gene therapy using one of these vectors; this is a problem associated with AAV-based vectors. One way this problem is addressed is to use immunosuppressive drugs in people who receive an AAV-based gene therapy.
An additional issue that has to be considered when discussing gene therapy involves its longevity. Some types of gene therapy, because they deliver or interact with genetic material, have the potential to be life-long treatments. This may at first sound great, but if someone had a bad reaction to a gene therapy there may be no way to fix it. This had been a problem with some cell therapies, where the delivered gene inserted into the patient’s own DNA ended up causing cancer. Subsequent cell therapies have used newer methods to ensure that any genes inserted into the patient’s own DNA do not get inserted into a region of the DNA that could cause cancer. As far as other types of gene therapy, such as gene replacement or RNA therapy, so far there have been no reports of long-term problems. This is likely due to the extensive amount of testing that gene therapy drugs undergo in animal and cell models before they are given to people and the careful monitoring of people who have had gene therapy drugs.
Regardless of these challenges, gene therapy continues to hold the promise of treating and maybe even curing diseases we never thought we could previously, including diseases such as STXBP1 disorder. Indeed, it is estimated that over the next 5 years that 40% of gene therapy drugs approved by the FDA will address rare diseases, including neurological disorders.