The Distinct Roles of Glutamatergic and GABAergic Neurons in STXBP1 Phenotypes
Introduction
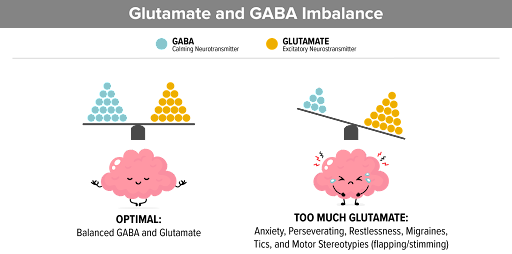
Glutamate and gamma amino butyric acid (GABA) are major neurotransmitters in our brain. Glutamate is an excitatory neurotransmitter, in that it tends to increase neuronal activity, while GABA is inhibitory. Neurons that use these neurotransmitters as their primary means of communication are referred to as Glutamategeric and GABAergic neurons. They work together to maintain proper brain performance; too little or too much of either can result in abnormal functioning and neurological problems (Figure 1). STXBP1 encephalopathy is characterized by a broad spectrum of neurologic problems that can include intellectual disability, seizures, movement disorder, developmental delay, autism, hyperactivity, anxiety, and aggressive behavior. Mutations in the STXBP1 gene impair glutamatergic and GABAergic neurotransmission. A newly released paper, from the laboratory of Mingshan Xue at Baylor, looks at the contribution of glutamatergic and GABAergic neurons to the different neurologic phenotypes associated with STXBP1 disorders (ref 1).
What Happens During a Glutamate/GABA Imbalance
Paper Summary
Presynaptic protein mutations are among the most common causes of neurological and neurodevelopmental disorders. The clinical features associated with such mutations are those usually associated with STXBP1: Seizures, movement issues, and lowered intellectual ability. While the function of these presynaptic proteins is generally known, how mutations lead to specific disease states remains unknown. Mouse models are very useful to help us understand the pathophysiology involved in STXBP1 encephalopathy and to help develop and test potential therapies. In a previous publication, the Xue lab described the creation of Stxbp1 haploinsufficient mice. These mice, which only have one working copy of the Stxbp1 gene and an approximate 50 percent decrease in Stxbp1 protein levels across the brain, demonstrated nearly all clinical features of STXBP1 encephalopathy, including cognitive impairments, epileptic seizures, motor dysfunction, developmental delay, anxiety-like behavior, hyperactivity, and aggression (ref 2). The mechanisms underlying haploinsufficiency the Stxbp1 gene leads to all of these neurological deficits is still unclear. What is known is that this loss impairs neurotransmission in all neurons including glutamatergic and GABAergic neurons. Thus, in order to test the roles of these neurons, the researchers generated mice where the loss of the Stxbp1 gene has been limited to either glutamatergic or GABAergic neurons and determined phenotypes for 3 areas- cognitive ability, epilepsy, and motor dysfunction.
Many tests of cognition and behavior (memory, anxiety, hyperactivity, nest building, aggression), motor function (hind limb grasping, balance), development, and seizure activity were performed to test the significance of each of the neurons in the disease. For example, haploinsufficiency in GABAergic neurons (which would result in greater glutamatergic tone in the brain) resulted in decreased body weight, disrupted nest building, reduced motor coordination, and increased anxiety and aggression toward other mice. Both GABAergic and glutamatergic haploinsufficient mice demonstrated dystonia (involuntary muscle contractions), though this was more severe in the GABAergic mice, seizures, though the type of seizures were different in the two groups of mice, and memory deficits, though again, the types of memory affected were different (Fig 2).
Stxbp1 haploinsufficiency in just the GABAergic neurons was sufficient to induce most of the clinical phenotypes associated with STXBP1 encephalopathy and observed in the Stxbp1 haploinsufficient mice. This led the researchers to believe that GABAergic neurons, and reduced inhibitory tone, play a major role in the disease mechanisms for STXBP1, with glutamatergic neurons being responsible for a smaller subset of phenotype expression. There were some deficits observed in the Stxbp1 haploinsufficient mice that were not observed in either the GABAergic or glutamatergic haploinsufficient mice and it is possible that other types of neurons may be involved in these phenotypes A topic of potential research for the future could look into dopaminergic and noradrenergic neurons, which are key in object detection.
Phenotypes of glutamatergic(Vglut2-cHet) and GABAergic(Vgat-cHet) neurons
Aside from adding to our understanding of the mechanisms underlying the clinical features of STXBP1 encephalopathy, this study has an impact on potential therapies. The Xue lab had previously demonstrated that restoring Stxbp1 gene function, using a viral-based gene therapy, in haploinsufficient mice improved numerous neurologic deficits, such as impaired memory (Fig 3). The current study implies that such an intervention will need to target GABAergic and likely glutamatergic neurons. The wide distribution of these neurons across the brain makes therapies such as gene replacement difficult. One advantage, however, is the potential for precise treatments. Based upon the symptoms a patient has, just one of the neurotransmitter systems could be targeted. This could be done through the use of small molecules, and allow treatments that can be low in risk and high in effect.
Memory Test Results on Mice
Sources/References
Kim JH, Chen W, Chao ES, Chen H, Xue M (2021). Glutamatergic and GABAergic neurons mediate distinct neurodevelopmental phenotypes of STXBP1 encephalopathy. bioRxiv:In Press.
Chen W, Cai Z-L, Chao ES, Chen H, Longley CM, Hao S, Chao H-T, Kim JH, Messier JE, Zoghbi HY, Tang J, Swann JW, Xue M (2020). Stxbp1/Munc18-1 haploinsufficiency impairs inhibition and mediates key neurological features of STXBP1 encephalopathy. eLife 9:e48705.
“TACA-Glutamate-and-GABA-Imbalance.” The Autism Community in Action (TACA), https://tacanow.org/family-resources/top-reasons-to-implement-a-gluten-free-casein-free-diet/attachment/taca-glutamate-and-gaba-imbalance/.
Campbell, Molly. “Missense, Nonsense and Frameshift Mutations: A Genetic Guide.” Genomics Research from Technology Networks, https://www.technologynetworks.com/genomics/articles/missense-nonsense-and-frameshift-mutations-a-genetic-guide-329274.